Trump’s EPA Pick Rejects Climate Science, Fights For Fossil Fuels
http://www.usatoday.com/story/news/nation-now/2016/12/09/trumps-epa-pick-rejects-climate-science-fights-fossil-fuels/95231986/

Alan: In the cartoon above, the people on the left would never to think to undergo surgery without anaethesia because they know implicitly -- and as a matter of scientific fact -- that would be crazy.
We also know, as a matter of fact, that global warming is real.
Furthermore, we know that global warming is caused, in large part, by human activity, most particularly the burning of fossil fuels. (Every gallon of gasoline produces 20 pounds of carbon dioxide. If you were to exhale carbon dioxide into a baloon, how much would that balloon weigh? How many balloons would "suck up" 20 pounds of gas?!? Yes, the math is as shocking as it seems.)
The Guardian: "John Oliver's Viral Video Is The Best Global Warming Debate You'll Ever See"
Jon Stewart Probes The Spectacular Idiocy Of Climate Change Deniers
Stephen Colbert On Climate Change
"Sea No Evil"

Conservative Christians And Global Warming
Pope Francis Believes Global Warming Is "Mostly Man Made"
Global Warming Deniers Are Full Of It. Here Comes The Enema!
 Every combusted gallon of gasoline releases 20 pounds of carbon dioxide.
Every combusted gallon of gasoline releases 20 pounds of carbon dioxide.
Imagine: 20 pounds of a gas.
How many balloons would that fill?
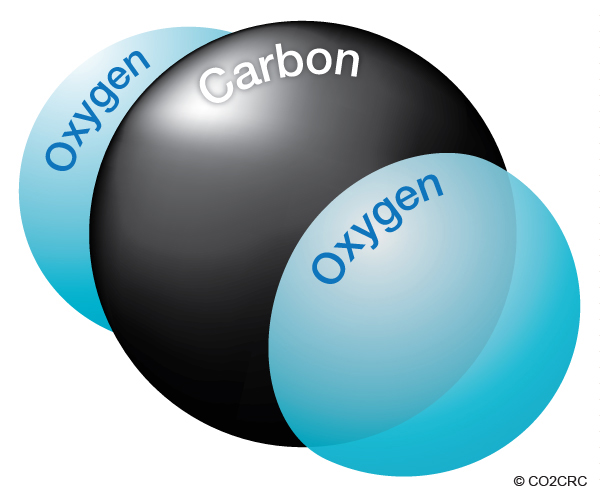
 A carbon atom has a weight of 12, and each oxygen atom has a weight of 16, giving each single molecule of CO2 an atomic weight of 44 (12 from carbon and 32 from oxygen).
A carbon atom has a weight of 12, and each oxygen atom has a weight of 16, giving each single molecule of CO2 an atomic weight of 44 (12 from carbon and 32 from oxygen).


Imagine: 20 pounds of a gas.
How many balloons would that fill?
It seems impossible that a gallon of gasoline, which weighs about 6.3 pounds, could produce 20 pounds of carbon dioxide (CO2) when burned. However, most of the weight of the CO2 doesn't come from the gasoline itself, but the oxygen in the air.
When gasoline burns, the carbon and hydrogen separate. The hydrogen combines with oxygen to form water (H2O), and carbon combines with oxygen to form carbon dioxide (CO2).
 A carbon atom has a weight of 12, and each oxygen atom has a weight of 16, giving each single molecule of CO2 an atomic weight of 44 (12 from carbon and 32 from oxygen).
A carbon atom has a weight of 12, and each oxygen atom has a weight of 16, giving each single molecule of CO2 an atomic weight of 44 (12 from carbon and 32 from oxygen).
Therefore, to calculate the amount of CO2 produced from a gallon of gasoline, the weight of the carbon in the gasoline is multiplied by 44/12 or 3.7.
Since gasoline is about 87% carbon and 13% hydrogen by weight, the carbon in a gallon of gasoline weighs 5.5 pounds (6.3 lbs. x .87).
We can then multiply the weight of the carbon (5.5 pounds) by 3.7, which equals 20 pounds of CO2!
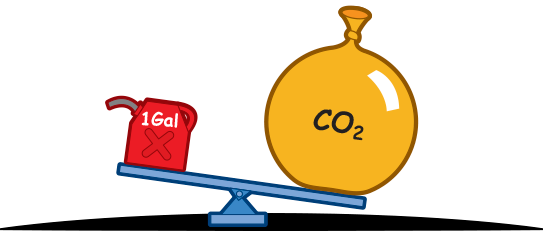

It's true.
The amount of carbon dioxide released by a gallon of combusted gasoline weights three times as much as the gallon of gas.


No comments:
Post a Comment